Abstract
Clonal hematopoiesis (CH) refers to the age-related expansion of specific clones in the blood system and manifests from somatic mutations acquired in hematopoietic stem cells (HSCs). Approximately 50% of CH variants occur in the gene DNMT3A. While DNMT3A-mutant CH becomes almost ubiquitous in aging humans, a unifying molecular mechanism to illuminate how DNMT3A-mutant HSCs outcompete their counterparts is still lacking. Here, we used interferon-gamma (IFNg) as a model to study the mechanisms by which Dnmt3a mutations increase HSC fitness under recurrent hematopoietic stress.
To represent the spectrum of DNMT3A variants found in humans, mouse genetic models were generated; Dnmt3a heterozygous (Vav-Cre; Dnmt3afl/+ = Dnmt3aHET) and homozygous (Vav-Cre; Dnmt3afl/fl = Dnmt3aKO) hematopoietic loss-of-function, and a knock-in model analogous to the hotspot point mutation most prevalent in AML (Vav-Cre; Dnmt3aR878H/+ = Dnmt3aR878).
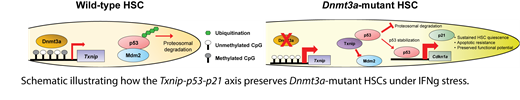
When Dnmt3a-mutant cells were competitively transplanted with wild-type (WT) competitor bone marrow (BM) cells and challenged with different inflammatory and proliferative stressors, Dnmt3aKO and Dnmt3aR878 HSCs were specifically resistant to the deleterious effects of IFNg on HSC self-renewal and clonal expansion. This insensitivity was also confirmed in a humanized mouse model where human CD34 + cord blood cells edited with DNMT3A-targeting gRNAs were xenografted into recipient mice and episodically exposed to human recombinant IFNg. DNMT3A mutant cells maintained their clone size, whereas AAVS1-targeted cells (control) were depleted over serial transplantation. These data suggest that Dnmt3a-mutant HSCs, mimicked DNMT3A-mutated human HSCs and are specifically resistant to IFNg-mediated depletion. One explanation for the observed resistance is that Dnmt3a-mutant HSCs have a fitness advantage under IFNg challenge. Therefore, we generated a novel mouse model to directly quantify the competition between Dnmt3a-mutant and WT HSCs. 10% donor BM cells (CD45.2; WT or Dnmt3a-mutant), 10% WT competitor BM cells (CD45.1/2; Ubc-GFP+) and 80% BM cells (CD45.1; IFNgr1KO; Rosa-M2-rtTA-IFNg) that express IFNg by doxycycline were transplanted into CD45.1 recipient mice. To normalize the effect of doxycycline, chimera made with 80% BM cells (CD45.1; IFNgr1KO; Rosa-M2-rtTA) were also transplanted into recipients. Our result from this transplantation experiment showed Dnmt3a-mutant HSCs resisted IFNg-mediated depletion due to an enhanced fitness advantage. Genetic ablation of IFNgr1 from Dnmt3a-mutant mice revealed that IFNg signaling is cell-intrinsically required by clonal expansion of Dnmt3a-mutant HSCs. In parallel, when HSCs were transplanted into IFNg-deficient recipient mice, clonal expansion of Dnmt3aKO HSCs but not WT HSCs was significantly compromised, suggesting IFNg signaling is also cell-extrinsically crucial for the clonal expansion of Dnmt3a-mutant HSCs in vivo. Mechanistically, DNA hypomethylation-associated over-expression of Thioredoxin-interacting protein (Txnip) in Dnmt3a-mutant HSCs was identified by coupling single-cell RNA-sequencing and Whole-Genome Bisulfite sequencing. The sustained Txnip levels in Dnmt3aKO HSCs led to p53 stabilization and upregulation of p21 under IFNg challenge, further correlated with a retained quiescence and resistance to apoptosis in response to IFNg exposure. Implementing biochemical studies, we observed Txnip mediated an enrichment of p53 at p21 promoter under IFNg exposure in Dnmt3aKO but not WT 32D murine myeloid cell line. Knocking down Txnip by shRNA normalized p53 occupancy at p21 promoter and rescued IFNg-associated p21 upregulation in Dnmt3aKO 32D cells. Functionally, knocking down Txnip and p21 re-sensitized Dnmt3aKO HSCs to IFNg-induced cell cycle activation and apoptosis. In vivo, down-regulation of p21 had no effect on WT HSCs in response to IFNg exposure, but it completely primed Dnmt3aKO and Dnmt3aR878 HSCs to IFNg-induced exhaustion in a transplantation experiment.
Taken together, our data highlighted a Txnip-p53-p21 pathway that preserves the functional potential of Dnmt3a-mutant HSCs under conditions of inflammatory stress, which suggests a novel mechanism to explain the increased fitness of Dnmt3a-mutant HSCs and supports rationale for developing interventions to mitigate expansion of pre-malignant clones as a method of blood cancer prevention.
No relevant conflicts of interest to declare.